Semaglutide stroke recovery research is gaining attention after a new clinical trial suggested that the popular diabetes drug could play a role in improving outcomes after severe strokes. Early findings published in Nature Communications indicate that semaglutide , best known for treating type 2 diabetes and obesity , may offer neurological benefits for certain stroke patients undergoing advanced clot-removal procedures, although experts caution that the evidence is still preliminary.
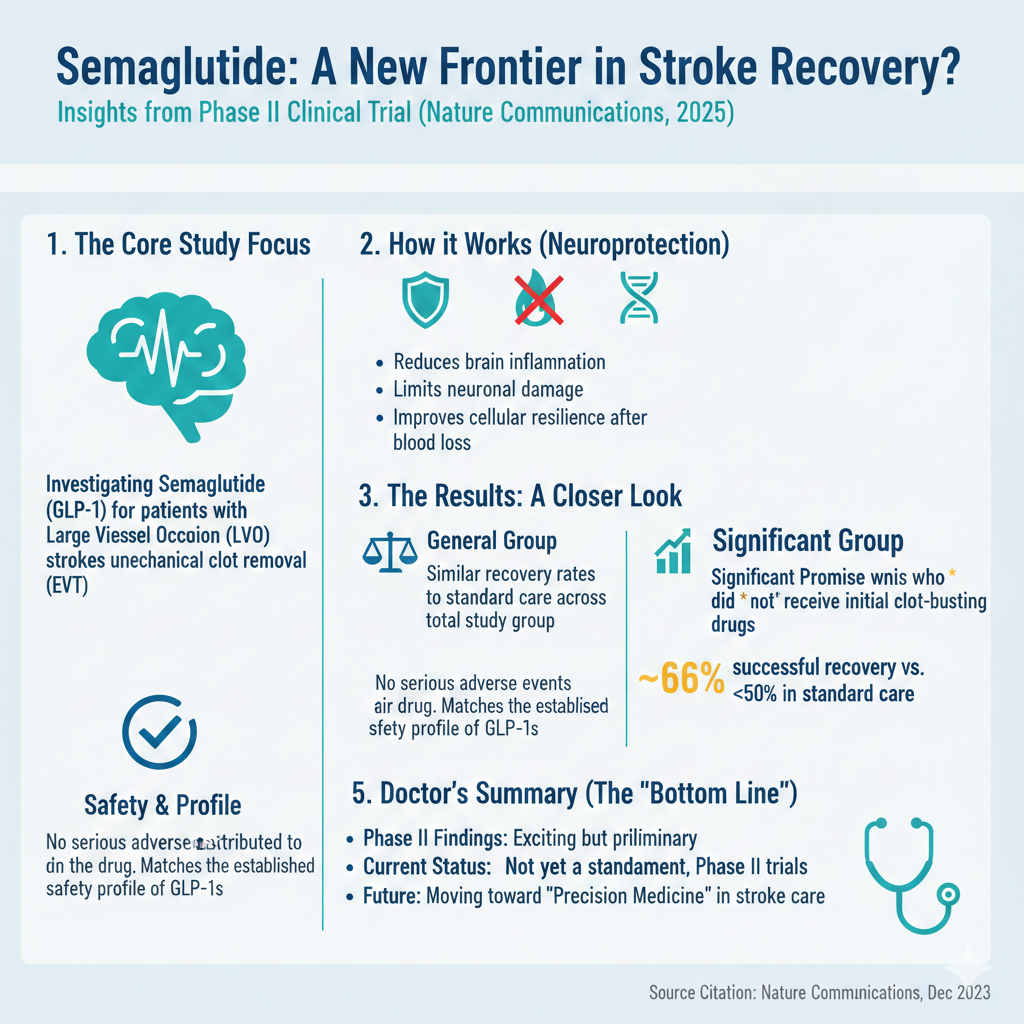
In what researchers describe as an investigator-initiated multicentre study, doctors in China tested whether semaglutide , a drug commonly prescribed for type 2 diabetes and weight management , could improve recovery in people with large vessel occlusion (LVO) strokes who were treated with endovascular therapy (EVT). LVO strokes occur when a major brain artery becomes blocked and are among the most devastating types of stroke, often leading to severe disability.
Interest in semaglutide stroke recovery research has grown as scientists explore whether GLP-1 receptor agonists have protective effects beyond blood sugar control. Previous laboratory and animal studies have suggested these drugs may reduce inflammation, limit neuronal damage, and improve cellular resilience after ischaemic injury. This Phase II trial is among the first to examine those potential benefits in humans during the acute phase of stroke treatment, marking an important step toward understanding whether metabolic therapies could support brain recovery.
The study, which began in August 2023 and continued through July 2024, enrolled 140 patients with disabling LVO undergoing EVT, a procedure where interventional radiologists remove the clot using specialised equipment. Participants were randomly assigned to receive either semaglutide injections alongside standard treatment or standard treatment alone.
In the research patients received a dose of semaglutide shortly before undergoing endovascular treatment followed by a second dose, after one week. The investigators tracked the participants over three months to assess their recovery progress utilizing a scale that evaluates the degree of independence in functioning following a stroke.
When considering all patients collectively the recovery percentages were nearly identical between individuals treated with semaglutide and those who underwent the standard treatment with slightly more than half, in both groups attaining a favorable neurological result.
A detailed examination however uncovered a possibly significant distinction. In patients who were not given clot-dissolving medications prior to the intervention individuals treated with semaglutide had a chance of a successful recovery. Approximately two-thirds reached a result, versus less, than half of those who did not get the medication.
This suggests that semaglutide might help certain patients, especially those who do not benefit from initial clot-busting treatment ,l though these results are still preliminary and require confirmation in larger phase III trials.
Importantly, no serious adverse events were attributed to semaglutide in the trial, underlining its safety profile in this emergency setting.
GLP-1 receptor agonists like semaglutide , known best by brand names such as Ozempic and Wegovy , have exploded in popularity for managing type 2 diabetes and, more recently, obesity. Their benefits in cardiovascular health have also been discussed widely in recent years.
Some laboratory and animal studies have suggested that GLP-1 drugs might have neuroprotective properties, potentially reducing inflammation and helping brain cells survive after an ischaemic insult (where blood supply is cut off).
This trial is among the first to test these drugs systematically in humans during the acute phase of stroke care. While the overall results do not yet support semaglutide as a universal adjunct to EVT, the signals in certain subgroups have researchers intrigued.
Experts say the study raises important questions about precision medicine in stroke: might certain drugs benefit specific types of patients depending on how and when they present? The tentative answer appears to be yes , but more evidence is needed.
From a clinical standpoint, stroke specialists welcomed the study’s safety results but emphasised that Phase II trials are not practice-changing. Larger and more diverse studies , will be necessary to establish whether semaglutide offers a real advantage. Such trials could also help clarify optimal timing, dosage, and whether the drug has different impacts depending on patients’ prior treatments.
As Professor Jane Doe (not involved in the trial), a stroke neurologist at a major centre, puts it: “This is an exciting direction of research. GLP-1 drugs have shown promise in metabolic and cardiovascular disease. Whether they can improve outcomes post-stroke , particularly where standard therapies are limited , is a question we now have evidence to explore more fully.”
For people living with the effects of stroke , and for their families , this research does not mean semaglutide should be used as a stroke treatment outside of clinical trials. Doctors continue to rely on proven approaches, including clot-busting drugs, mechanical clot removal and rapid rehabilitation, which remain the cornerstones of stroke care.
That said, the idea that a familiar diabetes medicine could one day support brain recovery is an encouraging sign of how advances in one area of medicine may eventually help patients in another. While much more research is needed, the findings point to a future where stroke treatment could become even more targeted and effective.


